Next: Example 13, Previous: Example 11, Up: Examples calorimetry [Contents][Index]
15.12 Example 12
A calorimeter of copper (105 g) contain 307 g of water at 23 C. If we add 95 g of ice at -4 C, What is the final temperature of the system in Celsius? (Specific heat of ice = 2090 J/kg*K, specific heat of water = 4187 J/kg*K, specific heat of copper = 390 J/kg*K, and heat of fusion to the water = 79.7 cal/g).
Solution with FisicaLab
Select the Thermodynamics group and, inside this, the Calorimetry and gases module. Erase the content of the chalkboard. And add one element Calorimetry, two elements Block, two elements Liquid, one element Change of state solid-liquid and one element Process. As show the image below (yellow circle contains the elements that represent the change of the ice):
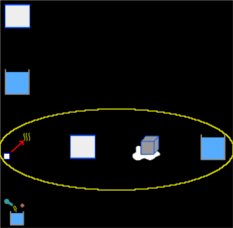
To the element Block, that represent the calorimeter of copper and gonna be called Copper, we have:
- Name
Copper
- m
105 @ g
- c
390
- Ti
23 @ C
- Tf
Tf @ C
To the element Liquid, that represent the water into the calorimeter and gonna be called Water, we have:
- Name
Water
- m
307 @ g
- c
4187
- Ti
23 @ C
- Tf
Tf @ C
Now, let’s assume that the ice melts completely and that the resulting liquid reaches a higher temperature at 0 C. Then to the second element Block, representing the ice in the temperature range of -4 C to 0 C and gonna be called IceA, we have:
- Name
IceA
- m
95 @ g
- c
2090
- Ti
-4 @ C
- Tf
0 @ C
Now to the element Change of state solid-liquid, representing the melting of ice and gonna be called IceB, we have:
- Name
IceB
- m
95 @ g
- cf
79.7 @ cal/g
- Sense
>
And to the second element Liquid, representing liquid water obtained by melting ice and gonna be called IceC, we have:
- Name
IceC
- m
95 @ g
- c
4187
- Ti
0 @ C
- Tf
Tf @ C
Now to the element Process, which represents the total change suffered by the ice water and gonna be called Ice, we have:
- Name
Ice
- Object 1
IceA
- Object 2
IceB
- Object 3
IceC
- Object 4
0
- Object 5
0
Note that the unknown data of the final temperature is the same in all elements, as must be. Therefore, to the element Calorimeter:
- Object 1
Copper
- Object 2
Water
- Object 3
Ice
- Object 4
0
Now click in the icon Solve to get the answer:
Tf = -1.154 C ; Status = success. Verify that the calculated temperature is within the range expected. Otherwise, some process or change never takes place because the energy isn't enough.
The warning message tells us that we must verify that the final temperature is within the expected range. Obviously the obtained final temperature is not within the expected range, since we assume that the ice has completely melted and the resulting liquid water took on a final temperature above 0 C. This suggests (as tells us the warning message) that some process is not carried out. The immediate possibility is that the energy of the calorimeter and water, is not enough to melt all the ice. So let’s assume that the ice reaches the 0 C, but only part of it melts. Thus removing the element Liquid, which represents the resulting water ice, and also delete its name from the element Process. Then the final temperature isn’t an unknown data, because if only part of the ice melts, then the final temperature is 0 C. The new unknown data is the mass of ice that melts. Thus, with this new hypothesis the elements to the problem are as shown the image below:
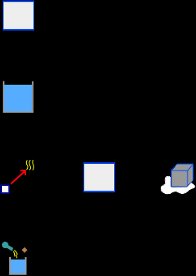
We must delete the element IceC in the element Process:
- Name
Ice
- Object 1
IceA
- Object 2
IceB
- Object 3
0
- Object 4
0
- Object 5
0
In the element Change of state solid-liquid, the mass is now the unknown data (we add the conversion factor to grams):
- Name
IceB
- m
m @ g
- cf
79.7 @ cal/g
- Sense
>
In the elements that represent the copper and its water, the final temperature is now 0 C:
- Name
Copper
- m
105 @ g
- c
390
- Ti
23 @ C
- Tf
0 @ C
- Name
Water
- m
307 @ g
- c
4187
- Ti
23 @ C
- Tf
0 @ C
Now click in the icon Solve to get the answer:
m = 89.037 g ; Status = success.
Therefor, the final temperature is 0 C, with only 89 g of melting ice.