Next: GNU Free Documentation License, Previous: Example 4, Up: Examples thermodynamics [Contents][Index]
17.5 Example 5
At a refrigerator with a coefficient of performance of 2.18 and 680 J of internal work, calculate the amount of heat (in Joules) extracted at the cold reservoir and the amount of heat transferred to the outside environment (the hot reservoir).
Solution with FisicaLab
Select the Thermodynamics group and, inside this, the Thermodynamics module. Erase the content of the chalkboard. And add one element Refrigerator, one element Applied heat, one element Heat extracted and one element Work. As show the image below:
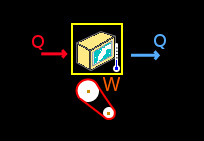
To the element Refrigerator set an arbitrary low temperature, we have:
- TH
H
- TL
100 @ C
- cop
2.18
To the element Applied heat:
- Q
Q
To the element Extracted heat:
- Q
Ex
And to the element Work:
- W
680
Then click in the icon Solve to get the answer:
Ex = 1482.400 J ; H = 271.170 C ; Q = 2162.400 J ; Status = success.