Next: Example 8, Previous: Example 6, Up: Examples calorimetry [Contents][Index]
15.7 Example 7
How much heat, in calories, should be removed to convert 25 g of molten lead at its melting point, in solid lead at 310 C? (Specific heat of the lead = 0.031 cal/g*K, heat of fusion of the lead = 5.54 cal/g, and melting temperature of the lead = 327 C).
Solution with FisicaLab
Select the Thermodynamics group and, inside this, the Calorimetry and gases module. Erase the content of the chalkboard. And add one element Process, one element Heat extracted (applied to the element Process), one element Change of state solid-liquid and one element Block. As show the image below (the yellow arrow are only to indicate the direction of the process):
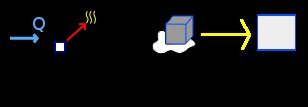
To the element Heat extracted, we have, using the conversion factor to calories:
- Q
Q @ cal
To the element Process (the elements Block and Change of state gonna be called Solid and Fusion respectively), we have:
- Name
0
- Object 1
Fusion
- Object 2
Solid
- Object 3
0
- Object 4
0
- Object 5
0
To the element Block, called Solid, we have:
- Name
Solid
- m
25 @ g
- c
0.031 @ cal/g*K
- Ti
327 @ C
- Tf
310 @ C
Note that the initial temperature is 327 C and the final 310 C. Now to the element Change of state solid-liquid, called Fusion, we have care with direction of the change of state (sign <). Then:
- Name
Fusion
- m
25 @ g
- cf
5.54 @ cal/g
- Sense
<
Now click in the icon Solve to get the answer:
Q = 151.675 cal ; Status = success.